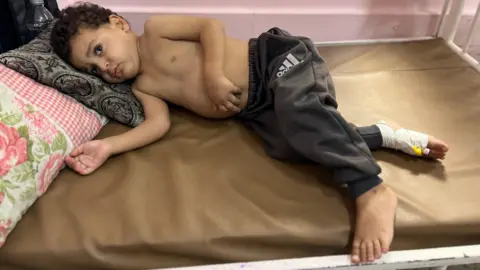
Members of the World Health Organization (WHO) have reached a pivotal agreement on a legally binding treaty aimed at strengthening global health security in preparation for future pandemics. This treaty seeks to address the chaotic conditions and resource competition that characterized the Covid-19 pandemic.
A key component of this new agreement focuses on the rapid sharing of data related to emerging diseases. This will enable scientists and pharmaceutical firms to expedite the development of effective treatments and vaccines. For the first time, the WHO will also maintain an overview of global supply chains for essential personal protective equipment (PPE), such as masks and medical gowns.
WHO Director-General Dr. Tedros Adhanom Ghebreyesus hailed the treaty as "a significant milestone in our shared journey towards a safer world," emphasizing that countries have demonstrated a commitment to multilateral cooperation despite global divisions.
This legally binding pact was finalized after three years of negotiations and marks only the second instance in the WHO's 75-year existence where such an international agreement has been established, the first being a tobacco control treaty in 2003. The pact is set for formal approval at the upcoming World Health Assembly next month.
Notably, U.S. negotiators were absent from the final sessions following former President Donald Trump's decision to withdraw the United States from the global health agency. Consequently, the U.S. will not be bound by the new treaty when its withdrawal becomes effective in 2026.
The agreement mandates that countries ensure worldwide availability of pandemic-related pharmaceuticals during future outbreaks. It stipulates that manufacturers allocate 20% of their production of vaccines, therapeutics, and diagnostics to the WHO, with a minimum of 10% to be donated and the remainder offered at affordable prices.
Additionally, member states have approved the transfer of health technologies to low-income countries, contingent on mutual agreement. This provision is aimed at facilitating local production of vaccines and medicines during health crises but has sparked controversy. Developing nations express discontent over past vaccine hoarding by wealthier countries, while nations with strong pharmaceutical industries are concerned that mandatory transfers could hinder research and innovation.
Central to the treaty is the proposed Pathogen Access and Benefit-Sharing System (PABS), designed to expedite data exchange among pharmaceutical companies. This system aims to empower firms to begin drug development more promptly in future health emergencies.