The launch of the first malaria treatment designed for babies, developed by Novartis, is set to commence in African nations soon, promising significant advancements in combating the disease which claims thousands of young lives annually.
Milestone in Global Health: First Malaria Treatment for Infants Approved
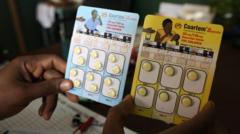
Milestone in Global Health: First Malaria Treatment for Infants Approved
A groundbreaking malaria treatment specifically for infants has received approval, aiming to address the high mortality rate among young children in Africa.
The approval of the first malaria treatment specifically designed for infants and very young children marks a significant milestone in global health efforts. This new drug, developed by Novartis, is expected to be distributed in African countries in the coming weeks. Previously, infants suffering from malaria had to rely on medications formulated for older children, which posed a risk of overdose due to their developing livers and differing body dynamics.
In 2023, malaria was responsible for approximately 597,000 deaths, with the majority occurring in Africa and about 75% of those fatalities involving children under five. The lack of appropriate treatments for the youngest patients led to what health experts have termed a "treatment gap."
The newly approved medicine, referred to as Coartem Baby or Riamet Baby in different regions, was developed in collaboration with the Medicines for Malaria Venture (MMV) — a non-profit organization supported by various governments and institutions, including the British, Swiss, and Dutch governments. The drug has been designed specifically for those weighing less than 4.5 kg (roughly 10 lbs), a demographic previously underserved in malaria care.
Novartis's chief executive, Vas Narasimhan, emphasized the significance of this development in the ongoing fight against malaria, expressing pride in the partnership that led to creating an optimized treatment for the world's tiniest and most vulnerable population.
MMV’s CEO, Martin Fitchet, hailed the approval as a critical step toward reducing the burden of malaria, especially among children, while Dr. Marvelle Brown from the University of Hertfordshire underscored the need for adequate healthcare access for those infants with compromised immune systems due to conditions like sickle cell disease.
As the drug is rolled out on a largely non-profit basis, health officials are hopeful that it will not only save countless lives but also enhance equity in healthcare access across regions most affected by malaria.