Bayer Ltd's recent recall of Yaz Plus contraceptive pills in South Africa has raised concerns over potential contraceptive ineffectiveness due to a packaging error, prompting health advisories.
Recall of Yaz Plus Contraceptive Pill in South Africa Due to Packaging Mix-up
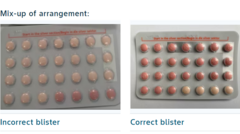
Recall of Yaz Plus Contraceptive Pill in South Africa Due to Packaging Mix-up
Affected women advised to seek medical counsel as Bayer Ltd takes corrective action
Regulators in South Africa have announced the recall of a specific batch of the Yaz Plus contraceptive pill, following a mix-up in packaging that could potentially render the contraceptive ineffective. The manufacturer, Bayer Ltd, reported that affected individuals should cease usage of the pills immediately and consult with healthcare professionals.
The issue arises from packs that contained 24 hormone-free inactive pills instead of the 24 active pills typically found in a regular Yaz Plus pack. These incorrect packs, identified under the batch number WEW96J with an expiration date set for March 2026, pose a risk as women taking them may be misled into believing they are protected from pregnancy.
Bayer Ltd emphasized that this situation only affects a limited number of packets from the identified batch. In collaboration with the South Africa Health Products Regulatory Agency, the company has confirmed that the underlying reason for the error has been thoroughly investigated and rectified.
To ensure safety, Bayer's recall notice states, "While only a limited number of packs from the respective batch is affected, as a precautionary measure, no tablets from these packs shall be used until you have consulted your healthcare practitioner, as they may potentially not provide the contraceptive protection you expect."
Individuals who have purchased the affected pills are urged to return them to pharmacies for replacements or refunds. The recall extends to healthcare professionals and organizations, including doctors, pharmacists, and wholesalers, who are also advised to return any remaining stock from the faulty batch.
In response to the incident, Bayer has created a helpline to address public inquiries, reaffirming their commitment to consumer safety. The company assures the public that no other batches of Yaz Plus are impacted by this issue, while the health of women who rely on this contraceptive option remains a priority.


















