Peter Marks, who played a pivotal role in the development of Covid-19 vaccines, submitted his resignation letter to the Department of Health and Human Services after being presented with a choice between resigning or facing termination. In his letter, Marks criticized the leadership of HHS Secretary Robert F. Kennedy Jr., implying a shift away from transparency and scientific integrity in favor of personal agendas.
Top FDA Vaccine Official Resigns Amid Leadership Shift

Top FDA Vaccine Official Resigns Amid Leadership Shift
Recent reports reveal that a prominent vaccine official at the FDA has resigned under pressure, indicative of the current administrative changes regarding public health communications.
Marks, who has led the FDA's Center for Biologics Evaluation and Research since 2016, expressed concern about a measles outbreak in Texas, linking it to a deterioration in public trust in science. The controversy arises from Kennedy's known vaccine skepticism and his recent overhaul of the HHS, which includes a significant reduction in workforce aimed at restructuring health communication within the agency.
Marks's departure highlights ongoing tensions within the FDA under Kennedy's leadership and raises alarms about the impact on public health policy and vaccine confidence amidst rising disease outbreaks in the country.
Peter Marks, M.D. resigns from the FDA, citing a troubling shift in public health leadership.
Marks' resignation underscores the growing divide over vaccine communications amid a measles outbreak and significant changes within the HHS.
Peter Marks, a leading vaccine official at the FDA, has resigned after expressing concerns about the new HHS Secretary Robert F. Kennedy Jr. and his approach to science and transparency. Marks' resignation letter highlighted the severity of an ongoing measles outbreak in Texas, linking it to a crisis of confidence in public health science. Kennedy's controversial leadership, marked by skepticism about vaccines and significant job cuts, raises important discussions about the future of health policies in the U.S.
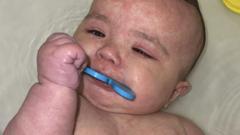
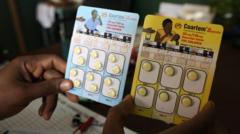
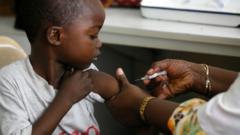
As of Friday, two fatalities were reported from the measles outbreak, which has seen over 500 cases arise nationwide, with Texas holding the majority. Marks's resignation, effective April 5, symbolizes a pivotal moment for the FDA at a time when public health information is increasingly politicized.
Kennedy's restructuring plans further complicate the narrative around vaccine advocacy and misinformation, calling into question the integrity of health communications under his stewardship. Meanwhile, the resignation showcases deep divisions in the current administration regarding vaccine safety and the role of scientific authority in shaping public health policy.
Peter Marks was a key player in the COVID-19 vaccine initiative under the previous administration and his departure signifies a contentious era in U.S. health governance as the fight against misinformation and the restoration of public trust are brought into stark relief.
In his resignation letter, Marks expressed concern about public health messaging amid rising vaccine hesitancy and health misinformation, illustrating the critical need for clarity and integrity in health communications.
The implications of these changes continue to unfold as figures like Robert F. Kennedy Jr. advocate for a new direction in public health, challenging established guidelines and raising questions about the future of vaccine confidence in a rapidly evolving health landscape.
Marks's departure highlights ongoing tensions within the FDA under Kennedy's leadership and raises alarms about the impact on public health policy and vaccine confidence amidst rising disease outbreaks in the country.
Peter Marks, M.D. resigns from the FDA, citing a troubling shift in public health leadership.
Marks' resignation underscores the growing divide over vaccine communications amid a measles outbreak and significant changes within the HHS.
Peter Marks, a leading vaccine official at the FDA, has resigned after expressing concerns about the new HHS Secretary Robert F. Kennedy Jr. and his approach to science and transparency. Marks' resignation letter highlighted the severity of an ongoing measles outbreak in Texas, linking it to a crisis of confidence in public health science. Kennedy's controversial leadership, marked by skepticism about vaccines and significant job cuts, raises important discussions about the future of health policies in the U.S.
As of Friday, two fatalities were reported from the measles outbreak, which has seen over 500 cases arise nationwide, with Texas holding the majority. Marks's resignation, effective April 5, symbolizes a pivotal moment for the FDA at a time when public health information is increasingly politicized.
Kennedy's restructuring plans further complicate the narrative around vaccine advocacy and misinformation, calling into question the integrity of health communications under his stewardship. Meanwhile, the resignation showcases deep divisions in the current administration regarding vaccine safety and the role of scientific authority in shaping public health policy.
Peter Marks was a key player in the COVID-19 vaccine initiative under the previous administration and his departure signifies a contentious era in U.S. health governance as the fight against misinformation and the restoration of public trust are brought into stark relief.
In his resignation letter, Marks expressed concern about public health messaging amid rising vaccine hesitancy and health misinformation, illustrating the critical need for clarity and integrity in health communications.
The implications of these changes continue to unfold as figures like Robert F. Kennedy Jr. advocate for a new direction in public health, challenging established guidelines and raising questions about the future of vaccine confidence in a rapidly evolving health landscape.